Background: Myelodysplastic syndromes (MDS) are heterogeneous hematological stem cell malignancies with a complex pathophysiology involving immune, genetic, and epigenetic changes induced by aging, oxidative stress, and genotoxicity. Dysregulated immune responses and aberrant inflammatory signaling have been identified as critical drivers of MDS development. Recent research has highlighted the significant association of the IL-33/ST2 signaling axis with tumorigenicity and tumor immunity in various cancers. Soluble ST2 (sST2), a transmembrane-free receptor induced by IL-33, negatively regulates the IL-33/ST2 signaling pathway, and elevated levels of sST2 have been reported to correlate with poor prognosis in malignant tumors. However, the specific level of soluble ST2 and the precise role of the IL-33/ST2 signaling axis in the context of MDS remain uncertain and require further investigation.
Method: This prospective study enrolled 89 newly diagnosed, untreated outpatient individuals with MDS to assess their clinical prognostic risk using the International Prognostic Scoring System-Revised (IPSS-R) and the International Prognostic Scoring System-Molecular (IPSS-M). A control group of 45 healthy donors (HD) was also included. The levels of various cytokines (sST2, IL-33, IL-2Rα, IL-6, IL-15) were measured using ELISA.
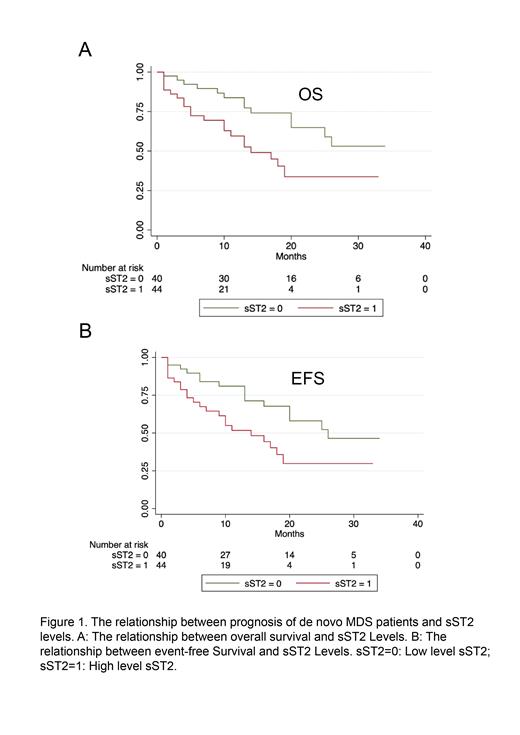
Result: Our study revealed that sST2 levels in MDS patients were significantly higher compared to HD (22.18 vs. 12.43 ng/ml, p = 0.0313), with no significant difference between low and high-risk groups. The Receiver Operating Characteristic (ROC) curve analysis showed an Area Under the Curve (AUC) of 0.6140 for sST2 (p = 0.0315, 95%CI 0.5199-0.7081). Using the maximum Youden index, the optimal cut-off value for sST2 (>14.71 ng/ml) was determined with a sensitivity and specificity of 51.69% and 73.33%, respectively. Progressive events include conversion to leukemia(n=8), transfusion-dependent occurrence(n=1) and death (n=29). Patients with higher sST2 levels in both high and low-risk groups demonstrated poorer Event-Free Survival (EFS) (p = 0.0193) and Overall Survival (OS) (p = 0.0130) (Figure 1). Further, multivariate analysis identified cytokine sST2(HR = 0.337, 95%CI 0.163-0.696, P=0.003), blast percentage(HR = 0.472, 95%CI 0.237-0.940, P = 0.033), and transfusion dependence(HR = 0.367, 95%CI 0.179-0.753, P = 0.006) as independent risk factors, indicating sST2's potential in predicting prognosis and adverse event risk in MDS patients. Furthermore, sST2 exhibited a significant correlation with other inflammatory cytokines (IL-2Rα, r = 0.4152, p = 0.0005; IL-6, r = 0.4897, p = 0.0013; IL-15, r = 0.3535, p = 0.0253). This suggests that sST2 plays an active role in immune system activation and chronic inflammation. Multiple attempts to detect IL-33 expression in the serum of MDS patients were unsuccessful, indicating the need for a more sensitive and specific assay. In our study, no statistically significant difference was observed in the level of sST2 between bone marrow and peripheral blood (P=0.2197), as well as before treatment and after chemotherapy (P=0.3965). These findings indicate that sST2 is an easily detectable and relatively stable indicator.
Conclusions: sST2 emerges as a significant, independent negative prognostic factor at the time of MDS diagnosis, offering valuable predictive insights into disease progression and a poor prognosis.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal